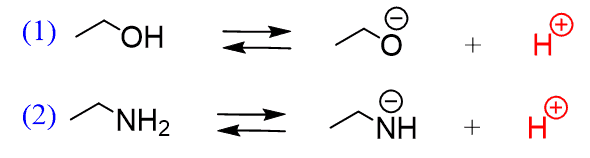
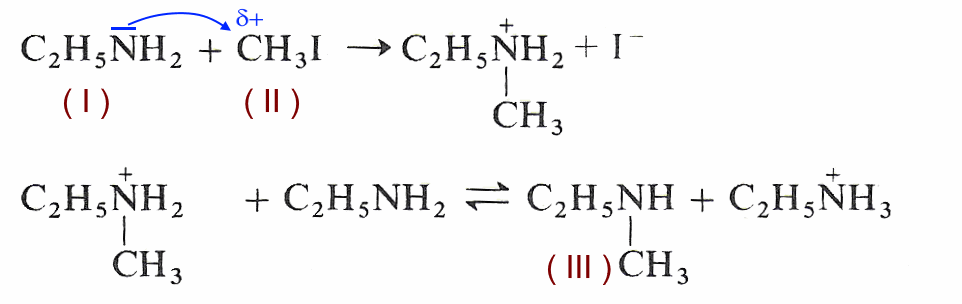Acid-base chemistry of aliphatic amines weak bases pKb Kb values why stronger than aomatic amines reactions with acids primary secondary tertiary balanced neutralisation equations organic nitrogen compounds organonitrogen molecules advanced A level

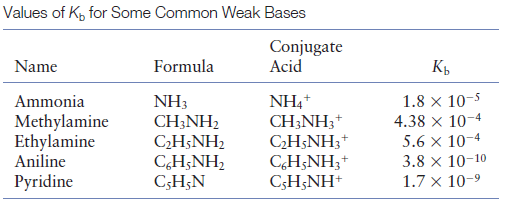
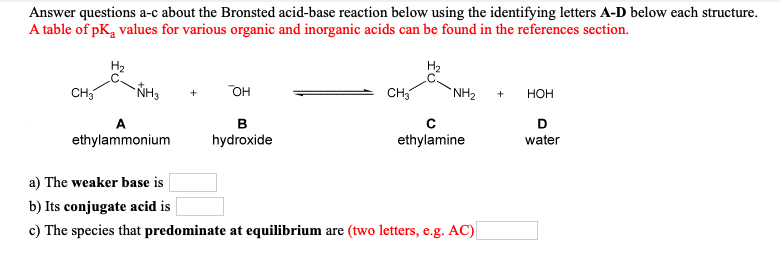
SOLVED:Which member of each pair is the stronger base? a. ethylamine or aniline b. ethylamine or ethoxide ion c. phenolate ion or ethoxide ion d. phenolate ion or acetate ion
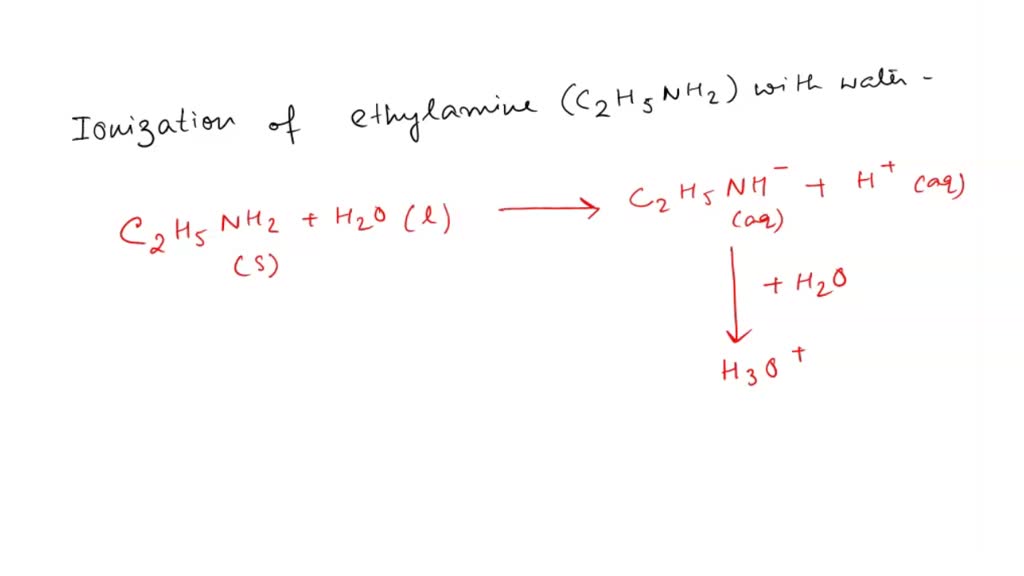
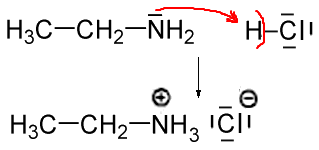
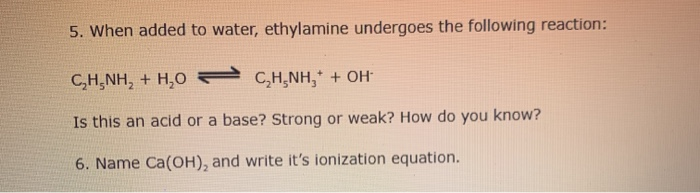
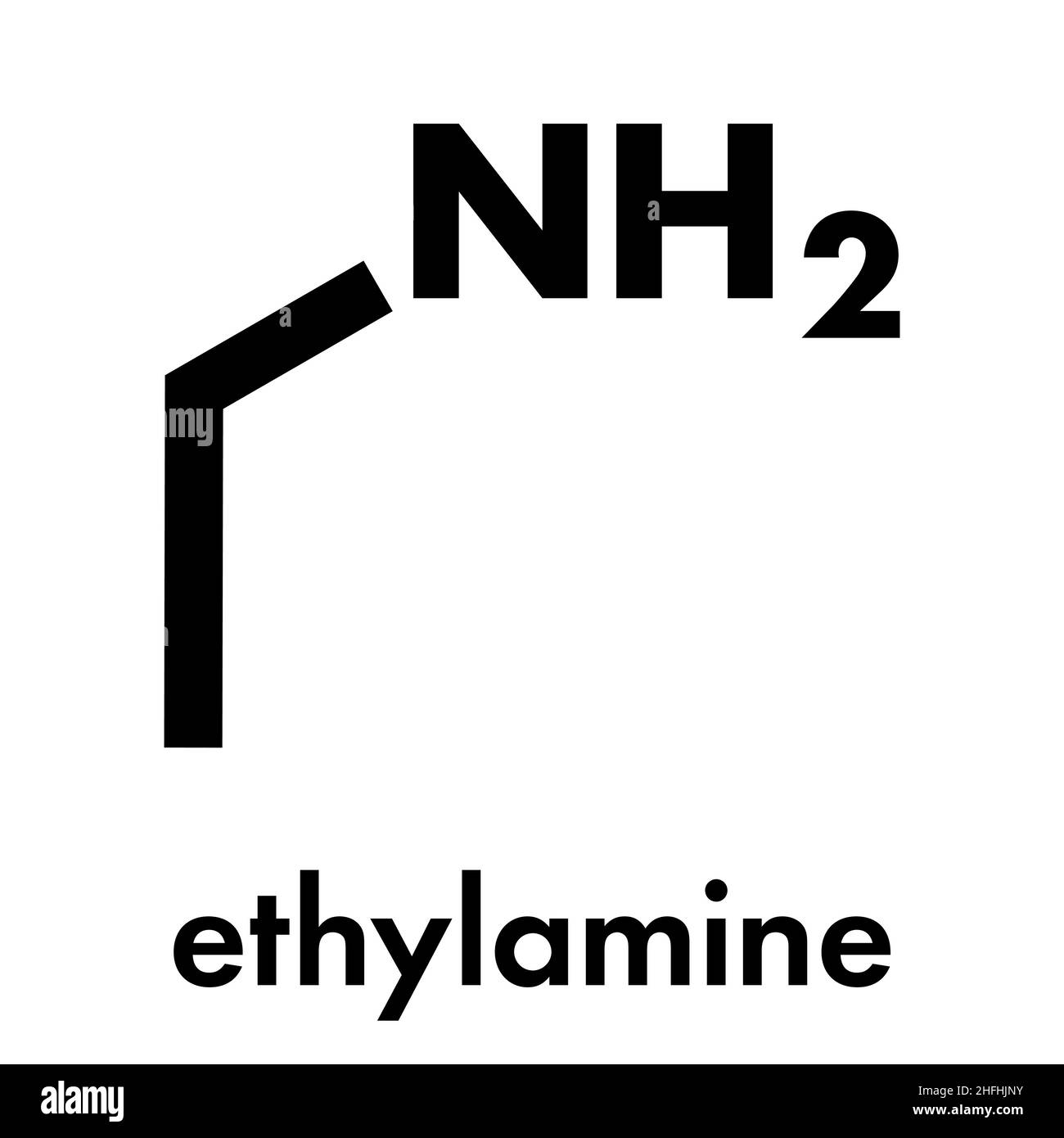
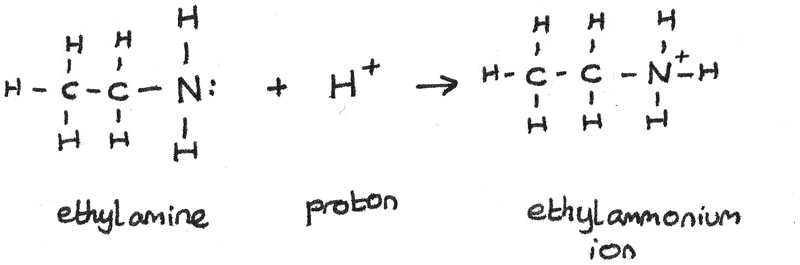
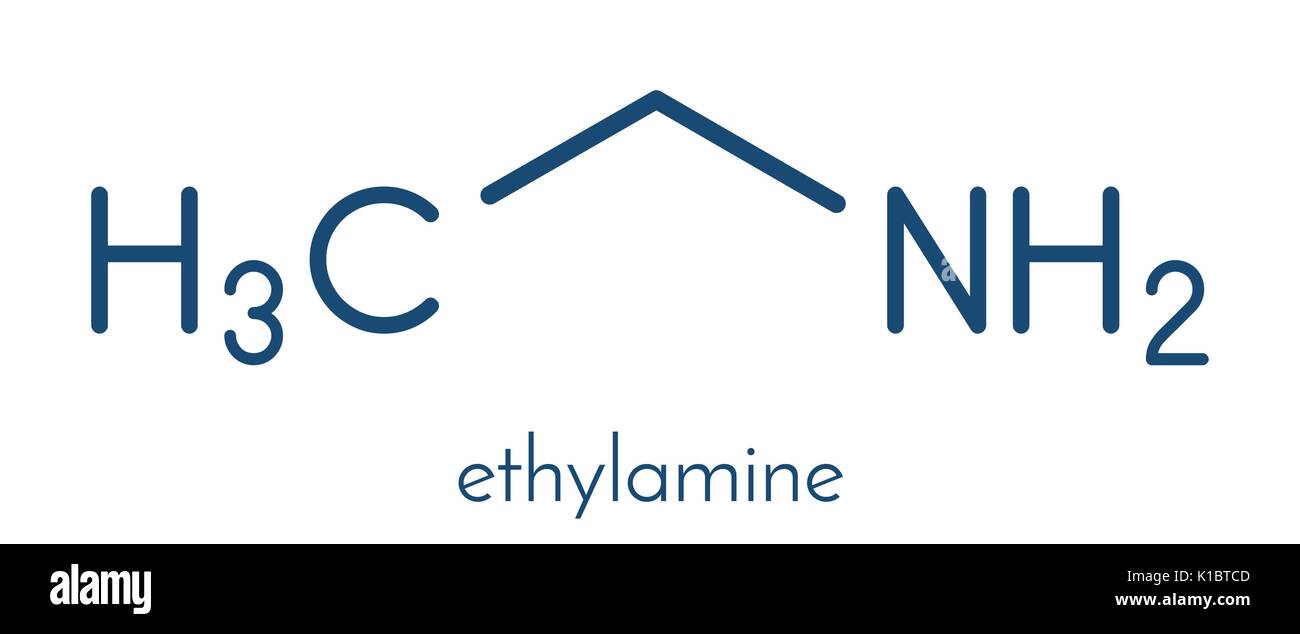
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water

Acid-base chemistry of aliphatic amines weak bases pKb Kb values why stronger than aomatic amines reactions with acids primary secondary tertiary balanced neutralisation equations organic nitrogen compounds organonitrogen molecules advanced A level